6.02 X 10^23
1 mole 602x1023 particles. Convert to Regular Notation 6021023 Mathway Algebra Examples Popular Problems Algebra Convert to Regular Notation 6021023 602 1023 602 10 23 Since the exponent of the.

Mole Day Moley Potter Anu Research School Of Chemistry
Exponents first - that means add 23 zeros to 10 then multiply it by 602 What the teacher is explaining is key things to not forget when working with exponents.

. 602 23 O D. To be specific at 602 is the precise time at which we celebrate Mole Day ideally in a chemistry lab or classroom somewhere. What is 6023 1023.
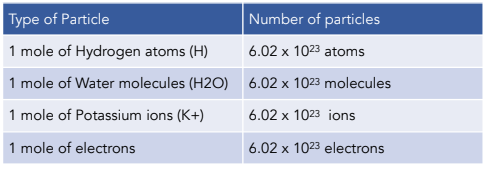
Thats where todays date comes in. You can have a mole of anything. This number is called Avogadro number.
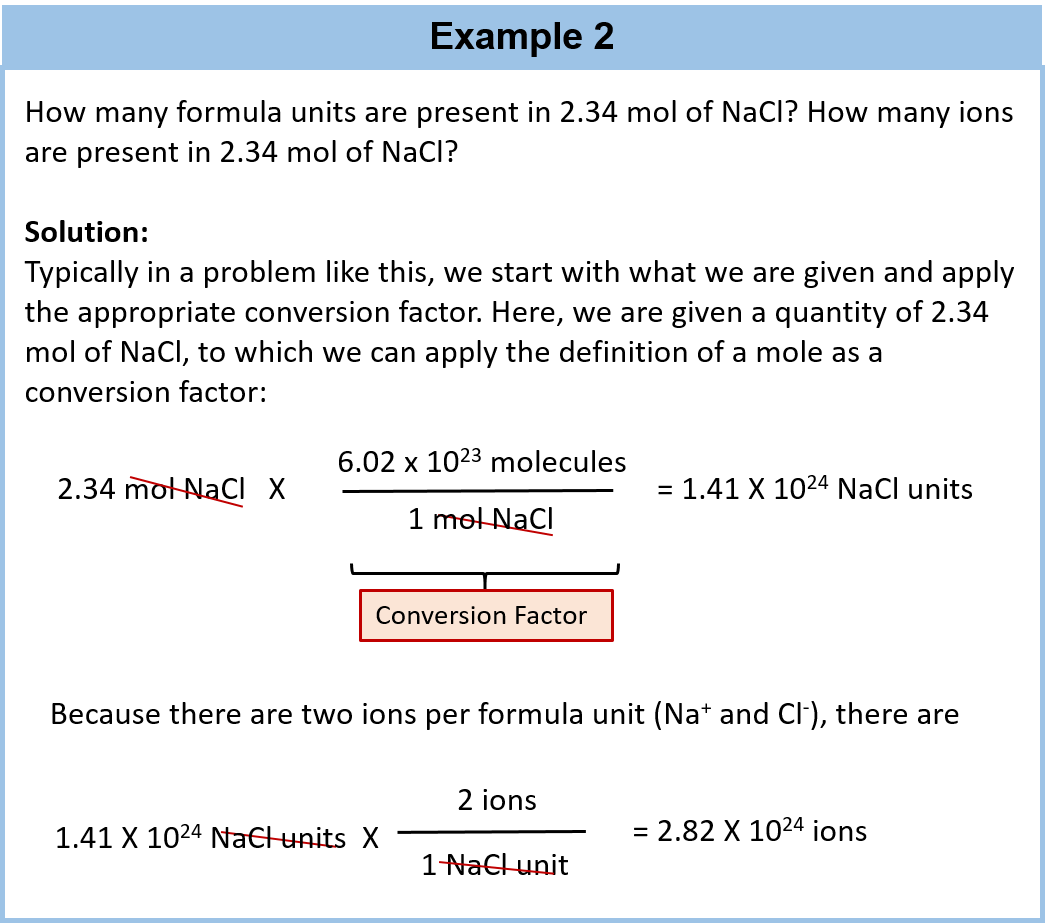
The amount of substance that contains 6021023. So they started by defining. You can have a mol of atoms.
Use a magnifying glass if you need to. Any substance that contains 6022 10²³ atoms is called one mole of that substance. 1g 602 10²³ amu suppose now we want to check number atoms of carbon in 12 grams of carbon because 12 grams is its molar mass 12g 602 10²³ 12 amu on both sides we.
I am rewriting the choices the way it appears i your. Thats what you find in the periodic table. This is a chemistry geek day.
An AMU is an atomic mass unit. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadros number of 602 x 1023 particles per mole. Its used to measures the number of atoms or molecules in a sample and it equals 602 x 10 23 a pretty hefty number.
The equation of 602 times 10 to the 23rd power is equal to 602000000000000000000000 or 602 followed by 21 zeros. 602 x 10-23 O C. Basically dealing with the weights of atoms and molecules individually is next to impossible.
A mole of a substance can be defined as. Theres 23 0s from the. The number of atoms in a 12g sample of C-12 B.
Look carefully at your picture. Hence one mole every elements contains.
Amount Of Substance The Mole And The Avogadro Constant A Level Chemistry Study Mind

Chemistry Mole Sample Kit Purchase A Mole Element Project For Your Chemistry Class Experiments At Teachersource Com

Answered One Mole Of Atoms Consists Of 6 02 X 10 23 Individua Physics

6 02 X 10 23 Stickers Cafepress
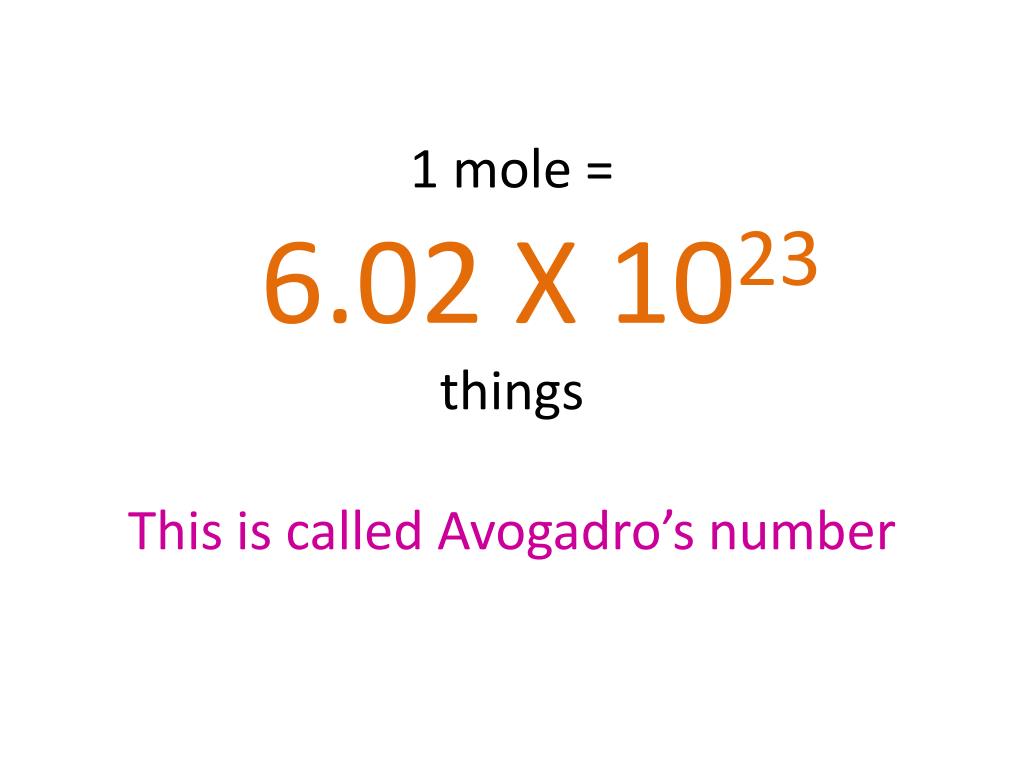
Ppt 1 Mole 6 02 X 10 23 Things This Is Called Avogadro S Number Powerpoint Presentation Id 4272623

Avogadro S Number Explained With Worked Examples

Mole Day Cake Mole Opoly 6 02 X 10 23 Mole Day Mole Chemistry Projects
Chapter 6 Quantities In Chemical Reactions Chemistry
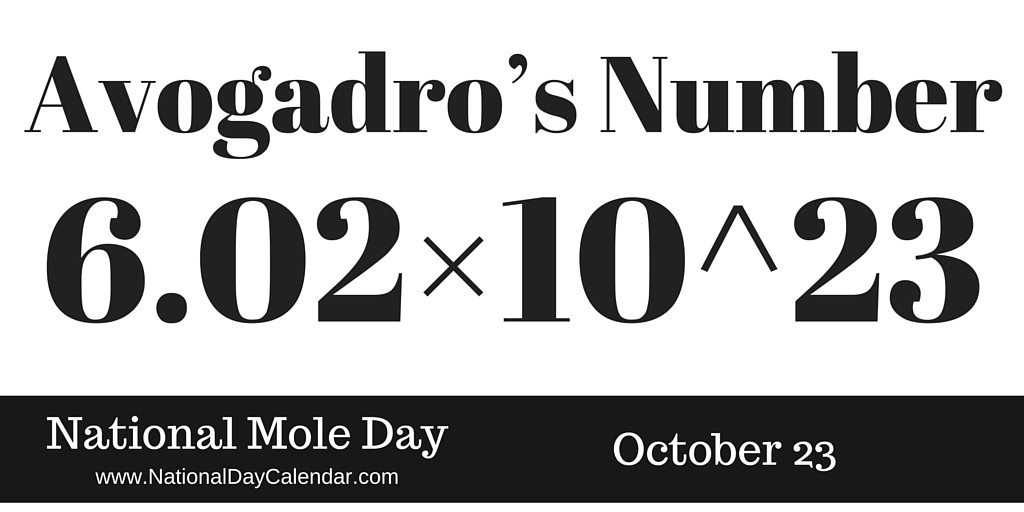
Avogadro S Number 6 02 X 10 23 National Mole Day
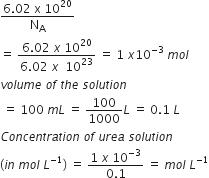
6 02 X 1020 Molecules Of Urea Are Present In 100 Ml Of Its Solution The Concentration Of Solution Is From Chemistry Neet Year 2013 Free Solved Previous Year Papers

Chapter 11 Matter Notes Mole Mol Is Equal To 6 02x10 23 The Mole Was Named In Honor Of Amedeo Avogadro He Determined The Volume Of One Mole Of Gas Ppt Download

Ppt Moles Powerpoint Presentation Free Download Id 3719148

Marc Connolly On Twitter It D Be Interesting To Try Chemistry Using Imperial Units Tbh Twitter

Moles Quantitive Chemistry Chemistry Gcse 9 1 Flashcards Quizlet

How Many Grams Of Ta Are There In 6 022 X 10 24 Atoms Of Tantalum Socratic
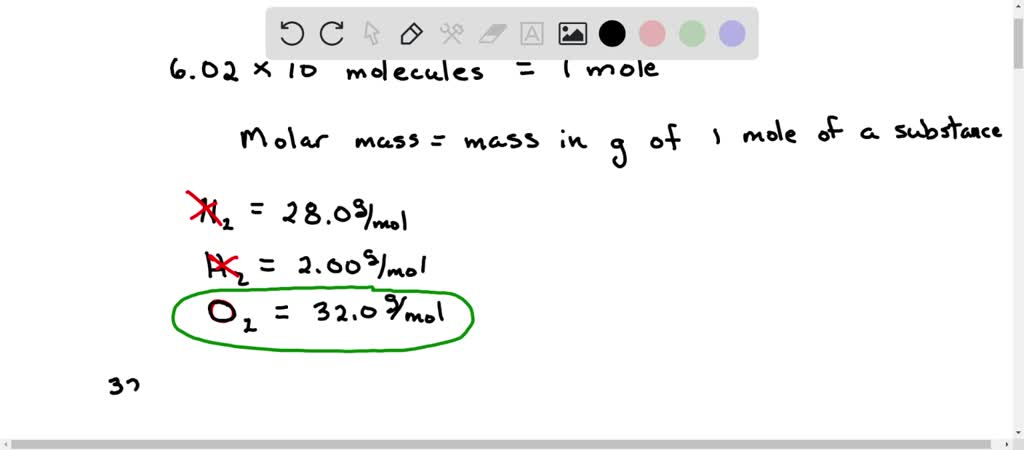
Solved Which Of The Following Molecules Contain 6 02 X 10 23 Molecules Group Of Answer Choices 14 0 G N2 None Of These 1 00 G H2 32 0 G O2 3 00 G H2o If

Avogadro Constant Wikipedia